Abstract
Background Expansion of blood cells derived from a single mutated blood stem cell - clonal hematopoiesis - often precedes leukemic transformation. However, the circumstances that promote or prevent transformation are poorly understood. Here, we investigate the evolution of clonal hematopoiesis into blastic plasmacytoid dendritic cell neoplasm (BPDCN), an aggressive blood cancer that often presents with malignant cells isolated to the skin (referred to as skin-only disease). We gain a better understanding of the influence of site-specific selective pressures and the role of TET2 mutations, which may inform interventions to prevent disease progression.
Methods We analyzed bone marrow and skin from 13 BPDCN patients using targeted, exome, and whole-genome sequencing, with germline controls. We performed multimodal single-cell sequencing (10x 3' v3.1 single-cell RNA-seq with genotyping of expressed variants) on bone marrow from 6 healthy donors (20,411 cells), 5 skin-only BPDCN patients with malignant cells in the skin but not conventionally detectable in marrow (36,018 cells), and 6 BPDCN patients with overt bone marrow involvement (30,582 cells). For functional studies, we targeted Tet2 using CRISPR/Cas9 in mouse blood progenitor cells immortalized by an estrogen receptor hormone binding domain fused to HoxB8, which undergo synchronized differentiation to myeloid and dendritic cells upon estrogen withdrawal. We used CellRanger, Seurat, RandomForest machine learning, and the R tidyverse package collection for bioinformatics.
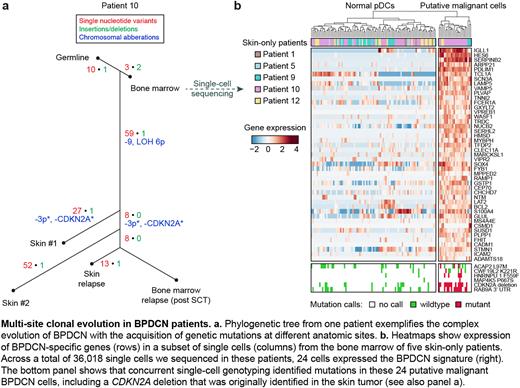
Results Genetics allowed us to reconstruct the evolution of BPDCN across bone marrow and skin (representative patient, panel a). Invariably, we detected founder mutations in marrow, including TET2 inactivation in 9/13 patients (two mutations, likely bi-allelic, in 5/9). Single-cell sequencing of marrow from 5 skin-only patients showed that pre-malignant mutant stem cells contributed to multiple lineages, and that TET2 mutated clones were myeloerythroid-biased. We generated a gene expression signature to identify rare malignant BPDCN cells in marrow of skin-only patients. In 3/5 patients, we identified rare cells (0.03-0.19%) with the transcriptional profile of fully transformed cells, and concurrent single-cell genetic analysis suggested that these cells originated from the skin tumor (panel b). To assess whether disseminated skin tumor cells establish bone marrow disease, we quantified mutational signatures: skin tumors and malignant BPDCN marrow cells in 5/5 patients harbored UV-induced mutations, likely acquired in a single skin-resident plasmacytoid dendritic cell (pDC) during malignant transformation. Finally, we edited Tet2 in mouse myelo-dendritic progenitors and tested the response to UV irradiation in vitro. In addition to observing that Tet2 inactivation increases dendritic cell commitment, the more striking result was a survival advantage of Tet2-mutated cells in the presence of UV (n = 6, fold change 1.803, P = 0.0012). These results highlight an unexpected function of TET2 that may predispose mutated pDCs to survive UV-induced DNA damage in the skin, leading to disease progression.
Conclusion The complex evolution of BPDCN traverses multiple anatomic sites prior to full transformation. This allows for accurate sequencing of oncogenic events. Disease evolution frequently starts with acquisition of a leukemia-associated mutation in a multipotent blood stem cell, leading to clonal expansion. In the most frequently observed scenario, bi-allelic TET2 inactivation promotes myeloerythroid bias, increases dendritic cell commitment, and confers resistance to UV-mediated cell death. Subsequent evolution of skin-resident mutant pDCs leads to a high burden of UV-induced mutations and ultimate transformation. Dissemination of malignant cells, which can be detected by integrating single-cell gene expression and mutation profiles, initiates "retrograde" bone marrow disease. Our discovery that multiple anatomic sites - and local selective pressures - play a role in disease progression provides a template to study multi-site evolution of clonal hematopoiesis.
Disclosures
Van Galen:ManaT Bio: Consultancy; Immunitas: Honoraria. Togami:Takeda Oncology: Current Employment. Chung:Janssen Pharmaceuticals: Current Employment. Verga:Cell Signaling Technology: Current Employment. Bernstein:HiFiBio: Consultancy, Current equity holder in publicly-traded company; Cell Signaling Technologies: Consultancy; Fulcrum Therapeutics: Consultancy, Current equity holder in publicly-traded company; Chroma Medicine: Consultancy, Current equity holder in publicly-traded company; Arsenal Biosciences: Consultancy, Current equity holder in publicly-traded company. Lane:Stemline Therapeutics: Research Funding; AbbVie: Research Funding; Qiagen: Consultancy, Honoraria; N-of-One: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal